With the implementation of the dual carbon strategy, the requirements of high-temperature industries for energy conservation and environmental protection are becoming more and more stringent, and the research and use of lightweight refractory materials are increasing. Mullite castables have excellent properties such as high load softening temperature, good thermal shock resistance, and strong erosion resistance. They are widely used in industrial furnace linings, ladle covers, and tundish linings. However, the dense aggregate and matrix lead to high thermal conductivity of the castable, which makes the heat loss of high-temperature thermal equipment large and the energy consumption high. Therefore, in order to achieve lightweight castables, it is necessary to develop refractory castables with high temperature resistance, low thermal conductivity, high strength and good thermal shock resistance.
Lightweight castables can be achieved through lightweight aggregates or lightweight matrices. Aggregates are the main contributors to the strength of castables. Only lightweight aggregates with good performance can produce high-strength and low-thermal-conductivity castables. Yi et al. used porous mullite microspheres, α-Al₂O₃ and SiO2 powder as the main raw materials, silica sol as a binder, AlF₃·HO₃O and VO₃O₅ as additives, and generated a large number of whiskers in the sample, thereby preparing a mullite castable with high strength and low thermal conductivity. Wang Yueyue et al. used spherical lightweight mullite aggregate, mullite fine powder and kyanite as the main raw materials to obtain a lightweight mullite castable with good fluidity, a volume density of 1.73g·cm⁻³, a thermal conductivity of 0.580W·(m·K)⁻¹, and good mechanical properties.
In the matrix part, lightweight design can also be carried out, that is, adding pore-forming agents to the matrix. Shi Yuheng et al. added polystyrene foam balls to the corundum-mullite castable. When the addition amount was 50% of the total volume of the slurry, the porosity of the castable was 61% and the thermal conductivity was as low as 0.220W·(m·K)⁻¹. Alumina hollow balls are often used as aggregates to prepare porous ceramics due to their high refractoriness and low thermal conductivity, but they are also a new type of inorganic pore-forming agent. When Wang Yu et al. added 30% (w) of alumina hollow balls to the mullite castable, the volume density of the castable was reduced to 1.96g·cm⁻³ and the thermal conductivity was 0.890W·(m·K)⁻¹.
In summary, lightweight design of the matrix and aggregate at the same time can obtain a castable with lower thermal conductivity while maintaining good mechanical properties, thereby reducing the energy consumption of high-temperature thermal equipment. Therefore, in this work, microporous mullite, mullite fine powder, alumina powder, and silica powder were used as the main raw materials, alumina hollow spheres were used as pore-forming agent, and cement was used as binder. The effects of the addition amount of alumina hollow spheres and heat treatment temperature on the properties of mullite castables were studied.
Test
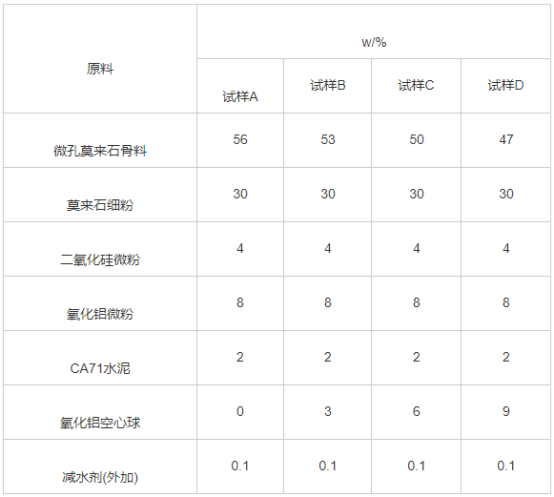
The test materials are: microporous mullite aggregates of 5-3, 3-1 and 1-0.086 mm, mullite fine powder ≤0.045 mm, silica powder, alumina powder, 2-1 mm alumina hollow sphere pore former, CA71 cement binder and sodium tripolyphosphate.
In this work, based on the closest packing theory and the Anderson formula, the performance of castables with different distribution moduli (n) was compared, and n=0.32 was selected for aggregate proportioning. The specific sample proportions are shown in Table 1. According to Table 1, the raw materials were weighed and mixed, and then put into a cement mortar mixer and wet mixed with water (adding about 13% mass fraction). Then pour it into the mold and cast it by vibration table. The sample size is 25mm×25mm×140mm. After curing at room temperature for 24 hours, demoulding is carried out. Then place it in an oven at 110℃ for 24 hours. Finally, the dried samples were heat treated at 1100 and 1400 °C for 3 h, respectively, and then taken out after cooling to room temperature.
According to GB/T2997-2015, the bulk density and apparent porosity of the sample were tested, according to GB/T3001-2017, the room temperature flexural strength of the sample was tested, according to GB/T5072-2008, the room temperature compressive strength of the sample was tested, and according to GB/T5988-2007, the heating permanent linear change of the sample was tested. According to YB/T4130-2005, the thermal conductivity of the sample after heat treatment at 1400℃ was tested (the hot surface temperature was 200, 400, 600, 800 and 1000℃ respectively). According to YB/T2206-1998, the thermal shock resistance of the sample was tested. After the sample was kept warm for 20 minutes in a muffle furnace at 1000℃, it was rapidly cooled in running water at 28℃ for 3 minutes, and then placed in the air for 10 minutes. This was repeated 5 times. After drying, the room temperature flexural strength of the sample was tested, and the strength retention rate was calculated to characterize the thermal shock resistance of the sample.
Results and discussion
2.1 Phase composition
As can be seen from Figure 1, the sample without hollow alumina spheres mainly contains calcium feldspar, mullite, corundum, etc., while the diffraction peak of the mullite phase in the sample with 9% (w) hollow alumina spheres is enhanced. This is because the raw materials such as alumina micropowder and silica micropowder in the matrix react at high temperature to generate products such as mullite and calcium feldspar. At the same time, an Al2O3-rich zone is formed at the interface between the hollow alumina spheres and the matrix, thereby increasing the amount of mullite generated.
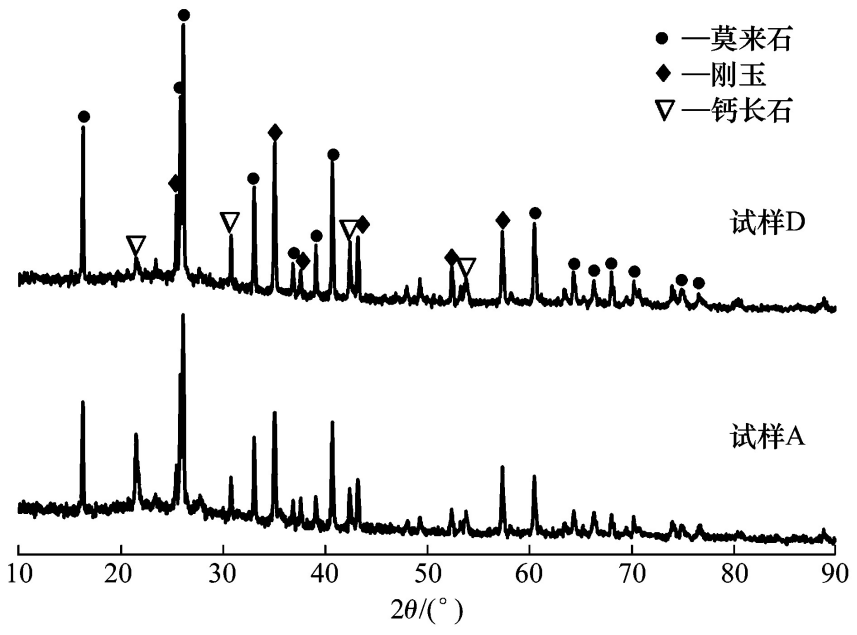
Figure 2 shows the apparent porosity and bulk density of the sample after heat treatment at 1400℃. It can be seen from the figure that with the increase in the amount of alumina hollow balls added, the apparent porosity of the sample gradually increases, while its bulk density decreases. When no alumina hollow balls are added, the porosity of the castable is 23.2%, indicating that microporous mullite can also provide a certain porosity for the castable. When the addition amount of alumina hollow balls is 9% (w), the apparent porosity is the highest, reaching 43.6%, and the bulk density is the lowest, which is 1.61g·cm⁻³. This is because the lightweight porous structure of the alumina hollow balls provides a large number of pores for the castable; at the same time, the alumina hollow balls promote the formation of mullite, causing volume expansion and increasing the porosity of the castable.
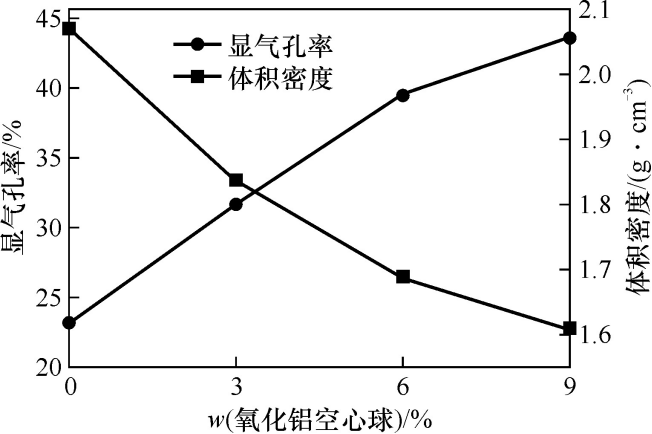
Figure 3 shows the heating permanent line change of the sample after heat treatment at different temperatures. It can be seen from the figure that with the increase of heat treatment temperature and the increase of the amount of hollow alumina balls added, the heating permanent line change of the sample is increasing. This is because the hollow alumina balls react with part of the matrix to generate mullite, causing volume expansion. As the temperature rises, the amount of mullite generated increases, and the heating permanent line change of the sample also increases. In addition, the interface area between the hollow alumina balls and the matrix is an Al₂O₃-rich area. The temperature of the liquid phase in this area is higher than that in other areas, and the liquid phase shrinkage rate is small, causing the heating permanent line change of the sample to increase.
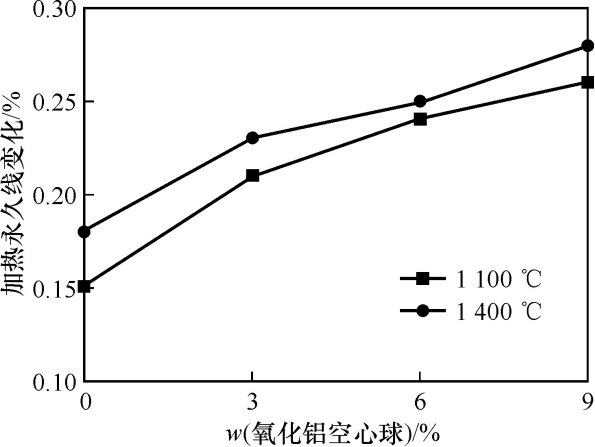
Figure 4 shows the room temperature strength of the samples after heat treatment at different temperatures. It can be seen from the figure that the room temperature strength of the sample decreases with the increase of the amount of alumina hollow balls added. At the same time, as the heat treatment temperature increases, the room temperature strength of the sample gradually increases. When the amount of alumina hollow balls added is 9% (w), the room temperature flexural strength and room temperature compressive strength of the sample after 1400℃ heat treatment are 11.9 and 47.1MPa, respectively. The strength of the sample after drying at 110℃ is mainly provided by the intrinsic strength of the mullite aggregate. However, the bonding strength between the matrix and the fine powder is not high, so the room temperature compressive strength is low at this time. After high temperature treatment at 1400℃, the sintering effect between the aggregate and the fine powder makes the particles in the castable more tightly bonded, and the alumina fine powder and the silica fine powder react to form mullite, which improves the strength of the castable. However, since the alumina hollow balls replace part of the aggregate, the porosity of the sample is increased, resulting in a decrease in mechanical strength. However, hollow alumina spheres increase the amount of mullite produced, and the porous structure of hollow alumina spheres can hinder crack propagation, so their strength decreases less.
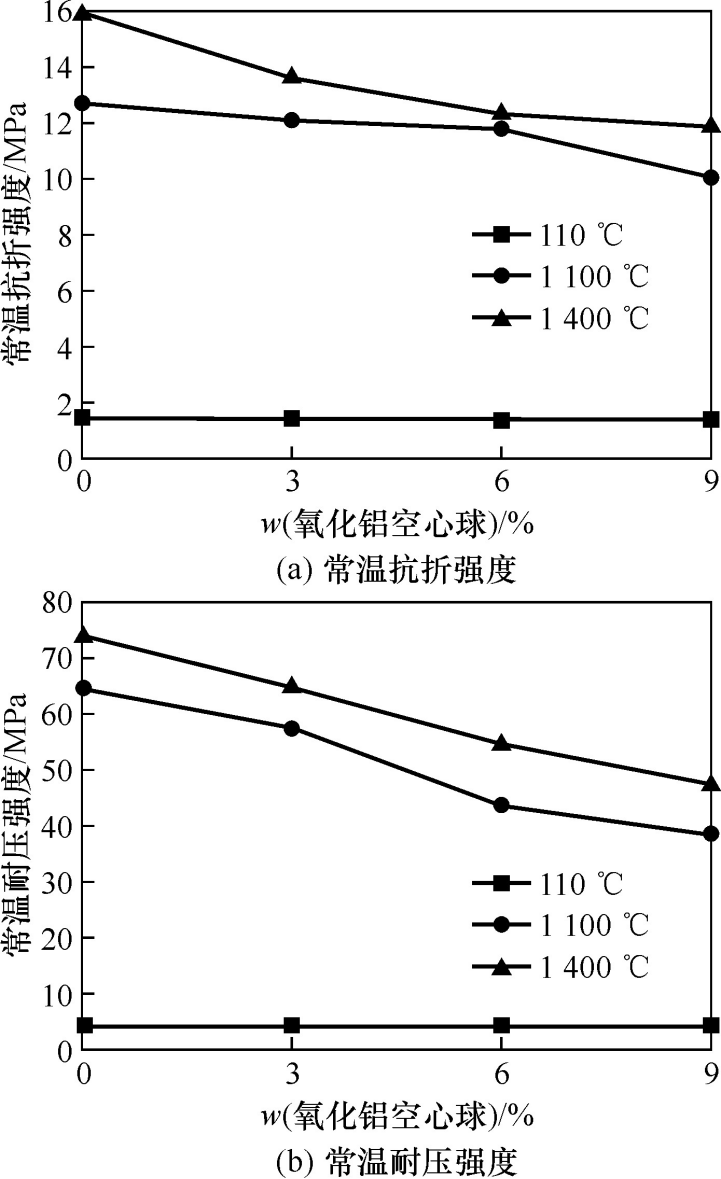
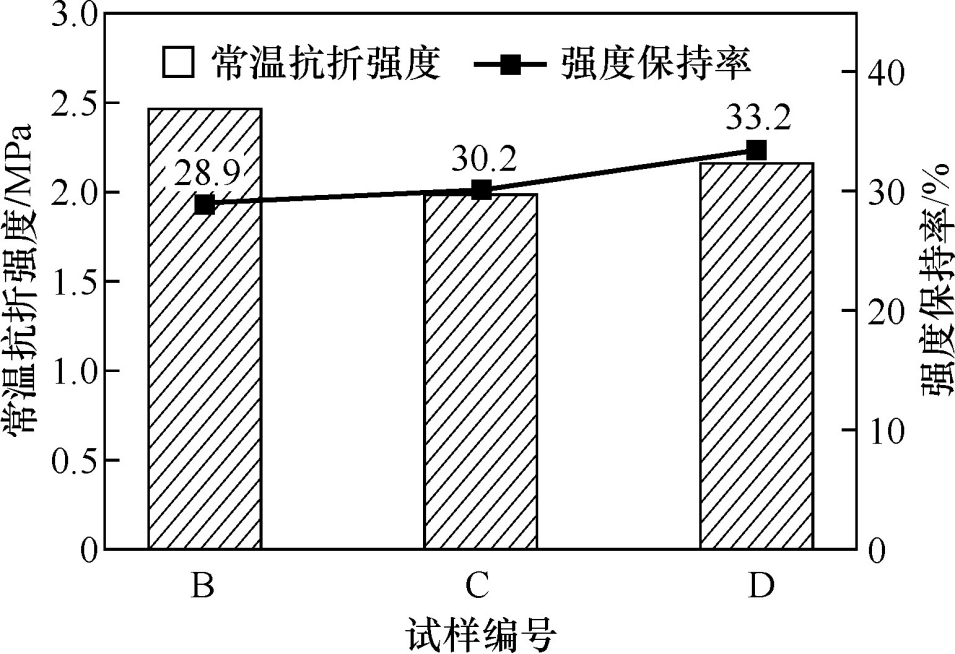
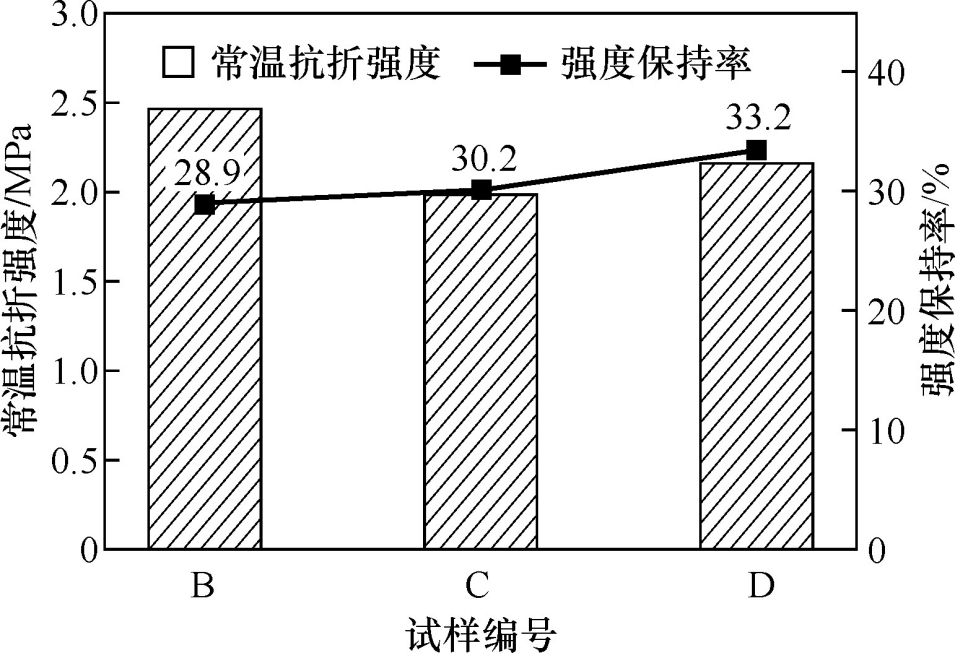
Figure 5 shows the room temperature flexural strength and strength retention rate of the sample after 5 thermal shocks at 1100℃. The sample without the addition of hollow alumina balls broke after 3 thermal shocks, and the remaining samples showed a small amount of cracks after 5 thermal shocks. It can be seen from the figure that with the increase in the amount of hollow alumina balls added, the room temperature flexural strength of the sample after 5 thermal shocks basically decreased, but the strength retention rate of the sample gradually increased. This is because the hollow alumina balls have a lightweight porous structure, and the microcracks in their pores can effectively alleviate the expansion of cracks, thereby improving the thermal shock resistance of the castable. In addition, the micropores in the microporous mullite aggregate are also conducive to increasing the crack expansion path and improving the thermal shock resistance of the castable.
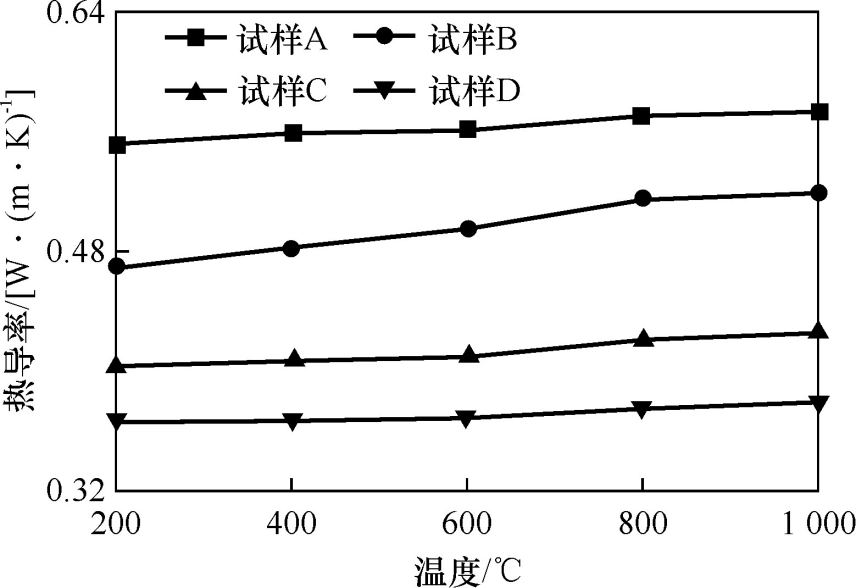
As can be seen from Figure 6, as the amount of alumina hollow spheres added increases, the thermal conductivity of the sample decreases. When the amount of alumina hollow spheres added is 9% (w), the thermal conductivity of the sample at 1000°C decreases to 0.380W·(m·K)⁻¹. This is because the porous structure of the alumina hollow spheres increases the porosity of the sample, thereby reducing the thermal conductivity. Microporous mullite itself also has many pores, which can also reduce the thermal conductivity of the sample.
Conclusion
Through the principle of closest packing, the component ratio of the castable is reasonably optimized to solve the problem of the difficulty in combining alumina hollow balls and lightweight mullite aggregate. With the increase of the amount of alumina hollow balls added, the apparent porosity of the sample increases significantly, the bulk density decreases, the thermal conductivity decreases significantly, the thermal shock resistance increases, and the strength decreases. When the addition amount of alumina hollow balls is 9% (w), the apparent porosity of the sample after heat treatment at 1400℃ is 43.6%, the bulk density is as low as 1.61g·cm⁻³, and the thermal conductivity at 1000℃ is as low as 0.380W·(m·K)⁻¹. The strength is still high, and the room temperature flexural strength and room temperature compressive strength are 11.9 and 47.1MPa respectively. In the context of energy conservation and emission reduction, it not only saves production costs, but also reduces energy consumption.
Feel free to contact us